Patients with microsatellite instable tumors benefit from specific treatments more than others. Therefore, early detection of instability becomes important. MicroSight MSI allows for such a result in less than two hours and provides a precise, personalised, and most importantly, beneficial treatment plan

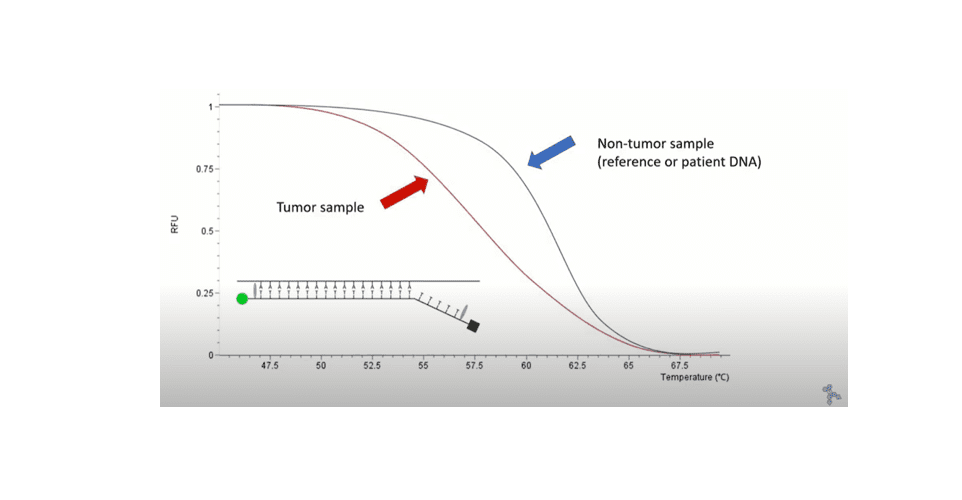
Microsatellite instability (MSI) is a genomic alteration in the length of microsatellites provoked by a deficient DNA mismatch repair system. MSI is known to occur in several types of cancer e.g., colorectal cancer (CRC), which accounts for 10.7% of newly diagnosed cancers globally (3’rd most occurring) according to World Cancer Research Fund’s data (2020). Identification of MSI in tumours is important as a predictive factor of response to different therapies, in particular to immune checkpoint inhibitors, and accordingly the need for MSI analysis of these patients has significantly increased. The MicroSight® MSI 1-step HRM Analysis assay analyses five well-described mononucleotide microsatellite sequences: BAT25, BAT26, NR22, NR24, and MONO27, by Real-Time PCR and HRM. The assay evaluates microsatellite length of patient tumour DNA to patient non-tumour DNA or a universal reference, which makes it a valuable stratification tool for assisting cancer treatment planning. After the Real-Time PCR and HRM, a semi-automatic analysis will be made by the instrument software giving objective results in less than 2 hours.
Advantages of MicroSight® MSI:
Results in less than two hours
Minimal hands-on time
Objective analysis – No biased data interpretation
One instrument analysis – Low risk of cross contamination
One of the fastest MSI Analysis in the world
MicroSight® MSI 1-step HRM Analysis assists in making the proper treatment plan for cancer patients. It; provides results in less than two hours, has flexibility through two versions, gives precise analysis of up to seven patients simultaneously, and does so all in one instrument.
2
Hour result time
2
Versions (Paired or Unpaired)
4 or 7
Patients
1
Instrument
Paired Samples
MicroSight® MSI 1-Step HRM Analysis assay offers an adaptive analysis of either paired or unpaired samples. Purified patient tumor and non-tumor DNA is added to individual 5-well PCR strip in the paired samples analysis. The strips are analysed in a BaseTyper™ 48.4 Real-Time PCR Instrument, where each run allows for quick analysis of up to 4 different patients simultaneously (4 sets of 2x5-well strips).
- The unique analysis of each patient’s specific DNA constitution
- Needs only 2 positive microsatellite loci for identification
- A quick, simultaneous 4-patient analysis
Instructions for Use:
Unpaired Samples
The unpaired sample analysis by MicroSight® MSI 1-Step HRM Analysis assay only needs tumour DNA from the patient, which is then compared to a Universal Reference DNA included in the kit. This allows for analysis of up to seven different patients simultaneously per Real-Time PCR run (7x patient 5-well strips + 1 Universal Reference 5-well strip).
- High throughput by use of Universal Reference DNA
- Needs 3 positive microsatellite loci
- A quick, simultaneous 7-patient analysis
- No requirement for matched uninvolved biopsy
Instructions for Use:
Product Information
PentaBase systems for analysis
The MicroSight® MSI 1-Step HRM Analysis assay is designed and validated for usage with the BaseTyper™48.4 Real-Time PCR Instrument
Fast and accurate qPCR
Minimal hands-on time required
Small, compact instrument with mobile capabilities


Order MicroSight® MSI 1-step HRM Analysis assay
Are you interested in our assays for analysing the stability/instability of five mono-nucleotide microsatellite loci? Find a local distributor, become our distributor or order your assay at PentaBase directly.









