MicroSight® MSI 1-Step HRM Analysis Assay
MicroSight® MSI is a simple and fast method for evaluating microsatellite instability (MSI) status of human DNA. The analysis is based on PentaBase’s proprietary INA® technology that enables fast and easy detection of microsatellite instability status by real-time PCR and high-resolution melt (HRM) analysis.
MicroSight® MSI requires very little hands-on time and enables evaluation of patient microsatellite status of purified human genomic DNA in less than one and a half hours for fast treatment guidance of cancer patients.
MicroSight® MSI can be used for analysis of both paired and unpaired tumour samples. Evaluation of unpaired tumour samples is done by comparison to an included universal reference. Each real-time PCR run can accommodate the analysis of up to 7 unpaired tumour samples.
- Used with BaseTyper 48.4 real-time PCR system
- Paired and unpaired sample analysis
- Semi-automatic objective analysis
- Results in less than 2 hours
- Minimal hands-on time
- CE IVD
- CE IVDR expected in Q4 2025
Product Details
Reference Number:
7201
Product Name:
MicroSight® MSI 1-Step HRM Analysis
Product Description:
PCR master mix and oligonucleotide mix for evaluation of MSI status of BAT25, BAT26, NR22 and MONO27, universal reference, positive and negative control
Format:
Ready-to-Use
Reactions:
16
Intended Use
Intended purpose
MicroSight® MSI 1-step HRM Analysis is an in vitro diagnostic medical device designed to qualitatively detect microsatellite stability (MSS) or instability (MSI) through the analysis of mutations in five mononucleotide loci (BAT25, BAT26, NR22, NR24, and MONO27) using a semi-automated real-time polymerase chain reaction (qPCR) followed by high-resolution melting (HRM) analysis. MicroSight® MSI 1-step HRM Analysis is specifically intended for use with human DNA extracted from biopsies from solid tumors, including formalin-fixed paraffin-embedded (FFPE) tissue.
The MicroSight® MSI 1-step HRM Analysis is intended to aid in diagnosing deficiencies in the mismatch repair (MMR) system through the analysis of microsatellite stability and instability in patients diagnosed with colorectal or endometrial cancer. MicroSight® MSI 1-step HRM Analysis is intended for use with patients already diagnosed with cancer who may benefit from additional genetic testing, to diagnose Lynch syndrome. Test results obtained using the product must be interpreted by healthcare professionals with other clinical findings, family history and laboratory data. It is not intended as a companion diagnostic device.
Intended user
The assay is intended for use by healthcare professionals, or qualified laboratory personnel, proficient in handling biological samples and specifically instructed and trained in the techniques of real-time PCR and HRM measurements.
Product Specifications
Procedure:
Real-time PCR HRM-based
Validated Real-Time PCR instruments:
BaseTyper™ 48.4 Quiet HRM Real-Time PCR System (PentaBase Ref. No. 754)
PentaBase technologies:
EasyBeacon™, SuPrimer™
Genomic targets:
BAT25, BAT26, NR22 and MONO27 microsatellites
Sample types:
Solid colorectal and endometrial cancer biopsies including formalin-fixed paraffin-embedded (FFPE) tissue samples
The sample tumor cell percentage:
At least 20%
Sample DNA extraction methods:
Relevant commercially avaliable solid biospy/FFPE DNA extraction kits
Sample input:
The assay requires 1–10 ng/µL of purified DNA isolated directly from the sample and not subjected to further chemical processing.
PCR run time:
Less than 2 hours
Instructions For Use
For the latest version of the Instructions For Use (IFU) – Click here –
Publications
One-instrument, objective microsatellite instability analysis using high-resolution melt
Bendixen et al. – PLoS One – 2024
Related files
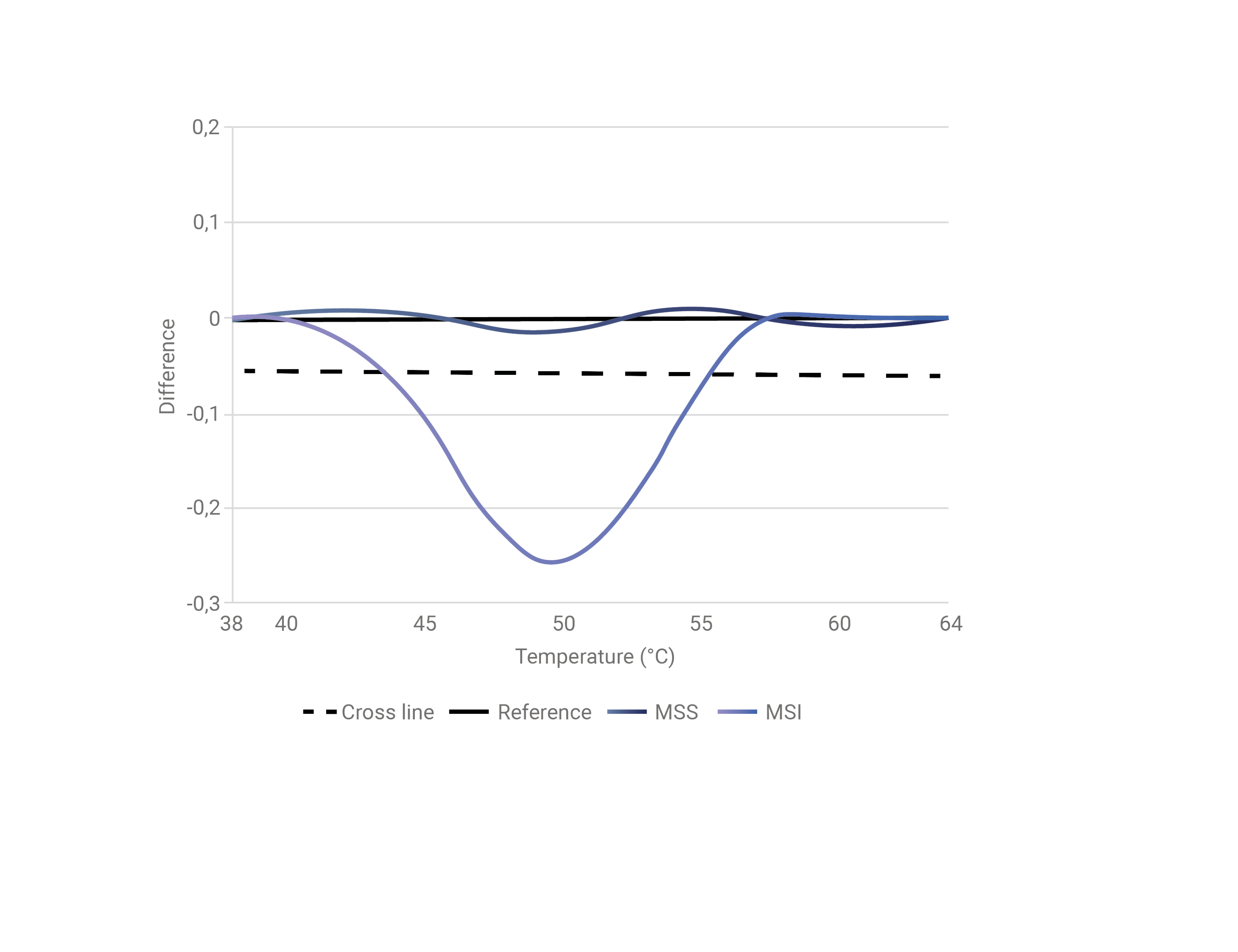
Semi-automatic Analysis of Well-establised Microsatellite Loci
MicroSight® MSI evaluates instability of the well-established microsatellite markers BAT25, BAT26, NR22, NR24 and MONO27 allowing for easy conversion from current methods based on capillary electrophoresis.
The BaseTyper software automatically calls each locus either stable (MSS) or unstable (MSI) based on the generated HRM difference plot and defined threshold for each locus.