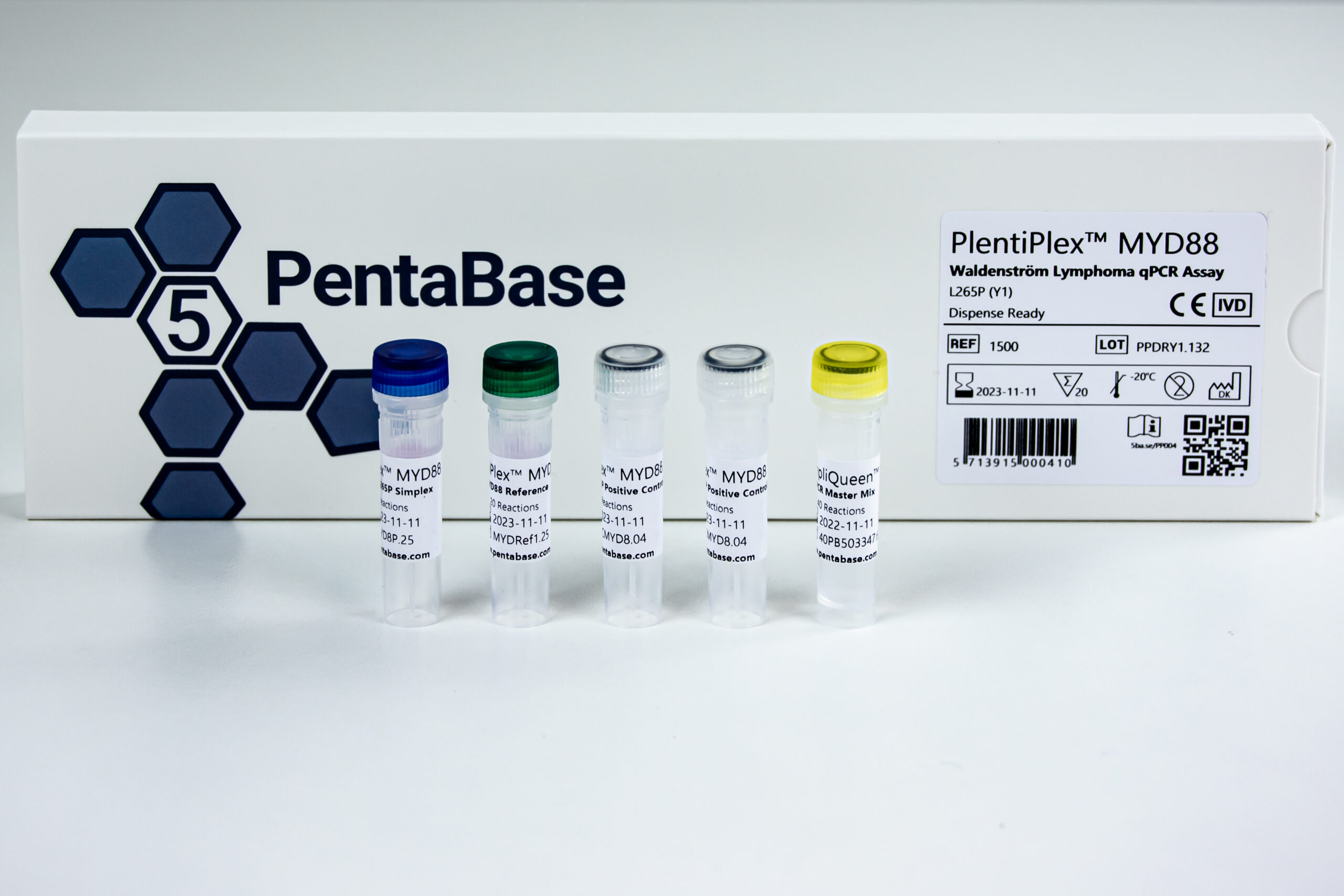
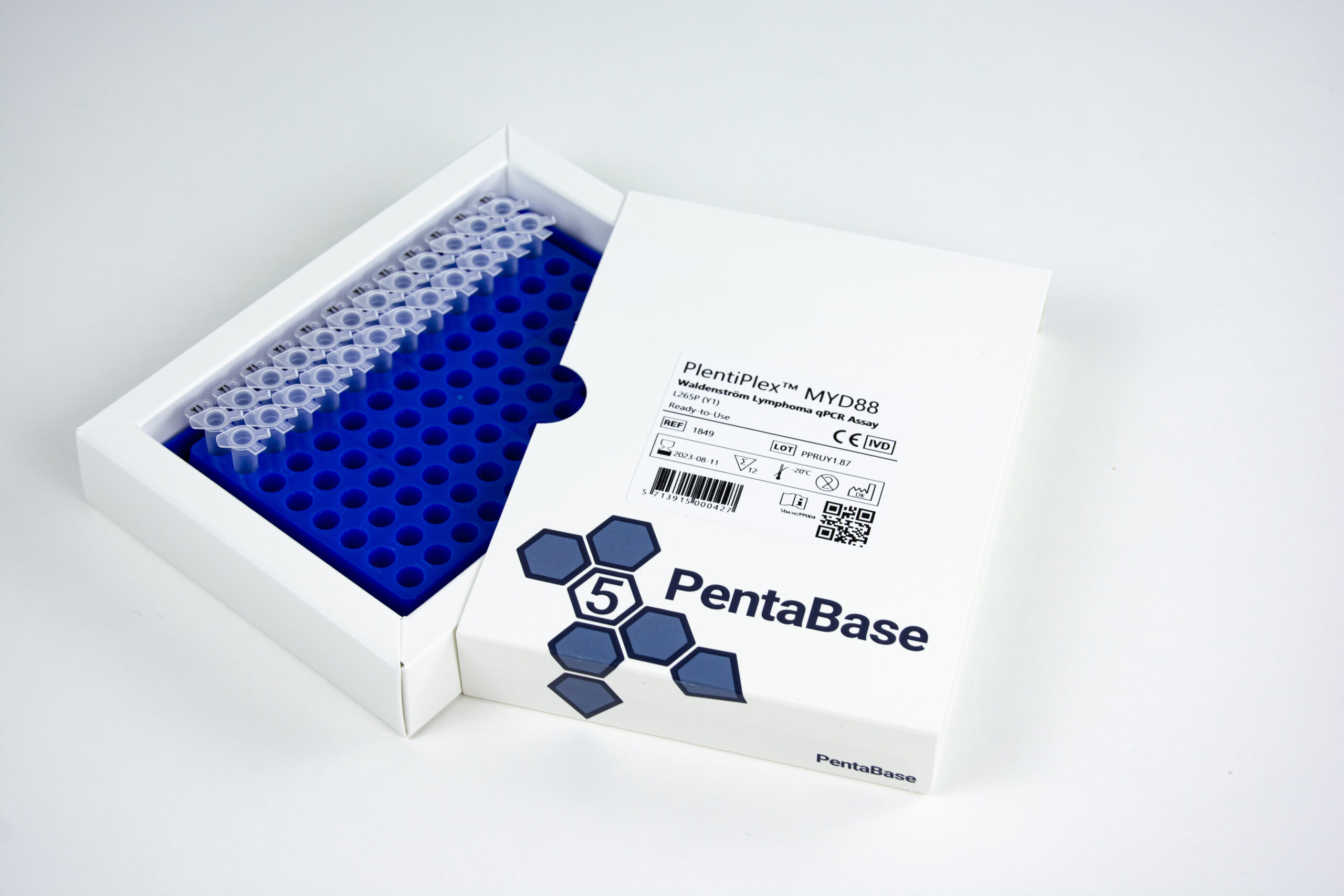
PlentiPlex™ MYD88 Waldenström Lymphoma qPCR Assay, CE IVD
The PlentiPlex™ MYD88 qPCR Assay combines high sensitivity with ease-of-use and is designed to work on standard real-time PCR equipment. The PlentiPlex™ MYD88 L265P assay is based on PentaBase’s novel and selective INA® technologies including the use of BaseBlockers™ that suppress false positive PCR signals from wild type templates.
- Open qPCR instrument procedure
- 0.6-0.75% MYD88 L265P LOD
- Results in less than 2 hours
- Based on INA® technology
- Minimal hands-on time
- CE IVD
- CE IVDR expected in Q4 2025
Product Details
Product Description:
PCR master mix and oligonucleotide mix for quantification of MYD88 L265P mutation, positive control
PlentiPlex™ MYD88Waldenström Lymphoma qPCR Assay:
20 reactions DR: 1500
12 reactions RTU: 1849
Intended Use
PlentiPlex™ MYD88 Waldenström Lymphoma qPCR Assay is a semi-quantitative real-time Polymerase Chain Reaction (PCR) assay intended for in vitro diagnosis of the genomic DNA (gDNA) change giving rise to leucine to proline mutation in codon 265 (L265P) of the Myeloid differentiation primary response 88 (MYD88).
Samples shall be obtained from Formalin-Fixed Paraffin-Embedded (FFPE) tissue or blood samples. The assay is used with real-time PCR systems and samples can be prepared using automated platforms or in manual workflows. The obtained results of PlentiPlex™ MYD88 Waldenström Lymphoma qPCR Assay are intended for identification of the presence of the MYD88 L265P, facilitating discrimination between Lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM) and non-Hodgkin lymphoma.
PlentiPlex™ MYD88 Waldenström Lymphoma qPCR Assay is intended for use by healthcare professionals or qualified laboratory personnel instructed and trained in the techniques of real-time qPCR as well as proficient in handling biological samples. Medical interventions based on results from this product requires medical authorisation.
Product Specifications
Procedure:
Real-time PCR-based
Compatible real-time PCR instruments:
Open platform design compatible with standard real-time PCR instruments
PentaBase technologies:
BaseBlocker™, HydrolEasy® Probe, SuPrimer™
Genomic target:
MYD88 L265P (c.794T>C)
Sample types:
FFPE and whole blood samples
The sample DNA extraction methods:
Relevant commercially avaliable FFPE or whole blood DNA extraction kits
Sample input:
1-10 ng/µL of purified DNA
PCR run time:
Less than 2 hours
Instructions For Use
For the latest version of the Instructions For Use (IFU) – Click here –
Publications
PlentiPlex™ MYD88 Waldenström lymphoma qPCR assay: A highly sensitive method for detection of MYD88 L265P mutation
Viscovo et al. – Int J Lab Hematol. – 2024
Related files
Assisting the Stratification of Lymphomas
The PlentiPlex™ MYD88 L265P assay is intended for in vitro diagnosis of the leucine to proline mutation in codon 265 of the Myeloid differentiation primary response 88 protein (MYD88 L265P) in genomic DNA (gDNA) samples. The results of the PlentiPlex™ MYD88 L265P assay assist clinicians in distinguishing patients with Lymphoplasmacytic Lymphoma/Waldenström Macroglobulinemia (LPL/WM) from those with other non-Hodgkin lymphomas.